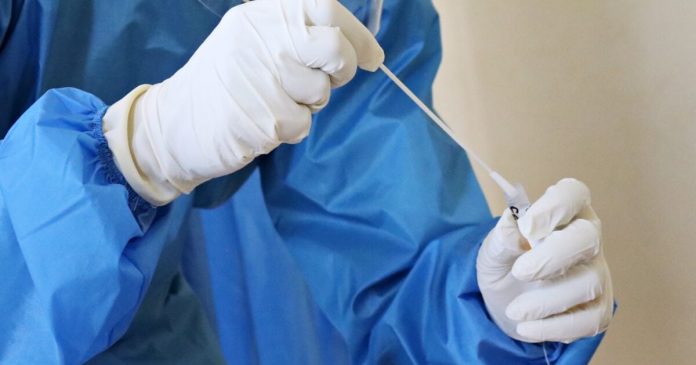
One brand name of at-home COVID-19 test is being remembered in the middle of a caution from the Food and Drug Administration that the test might cause a bacterial infection.
SD Biosensor, Inc. Pilot COVID-19 At-Home Tests, dispersed by Roche Diagnostics need to be tossed out, according to a brand-new release on the FDA’s site, which notes 44 impacted lot numbers.
The FDA reported that about 500,000 tests were dispersed to CVS Health. Another 16,000 tests were dispersed to Amazon. The FDA stated it is unclear the number of systems were offered.
None of the tests that have actually been remembered were dispersed by the federal government through COVID.gov/ tests– Free at-home COVID-19 tests or any other screening program, the FDA stated.
Recall Notice– SD Biosensor, Inc. Demands Discontinuation of Use and Disposal of Specific Pilot
COVID-19 At-Home Tests in the United States Due to Microbial Contamination in the Liquid Buffer Solution https://t.co/xb6GXGrOv8 pic.twitter.com/Ub9evKKHaJ
— U.S. FDA Recalls (@FDArecalls) May 5, 2023
The FDA caution stated that anybody who purchased a package that has the afflicted lot numbers noted on the FDA site must toss the whole set in the garbage. The FDA cautions versus direct exposure to a liquid option in the package and stated that if contact happens in between the liquid and either skin or eyes, the point of contact must be flushed with water.
The liquid in the impacted lots has actually been polluted with organisms such as Enterococcus, Enterobacter, Klebsiella and Serratia types of germs.
Although the liquid remains in a tube in the packages, contact is possible while utilizing the set.
The FDA desired that infection from the germs “might trigger disease in individuals with weakened body immune systems or those with direct exposure to the infected liquid option through basic handling, unintentional spills, or abuse of the item.”
That’s not all, the FDA stated. Test outcomes might likewise be unreliable
The FDA cautioned that both incorrect unfavorable tests, in which the individual has actually COVID-19 however it is not discovered, and incorrect positives, in which a person does not have actually COVID-19 however is revealed on the test to be contaminated, are possible.
The FDA likewise encouraged medical professionals who have actually utilized the test within the previous 2 weeks to have the private re-tested.
” SD Biosensor Inc., the maker of the Pilot COVID-19 At-Home Test, notified Roche that this problem was determined throughout regular quality control screening. Possibly hazardous germs were discovered in the liquid buffer service,” Roche stated in a declaration, according to CBS
Evie Baik, an agent of SD Biosensor, stated in a declaration that the infection originates from a concern with basic materials from a provider.
” To date, no such disease has actually been reported and to date no influence on efficiency has actually been validated,” Baik stated.
This is not the very first recall of the year for at-home COVID-19 tests. In February, more than 56,000 COVID-19 antigen quick tests were remembered, according to Fox Business
The Skippack Medical Lab SARS-CoV-2 Antigen Rapid Test Kits produced by Universal Meditech Inc., “possibly might lead to incorrect test outcomes due to absence of efficiency assessment by the FDA,” the recall notification stated.
This post appeared initially on The Western Journal
The post COVID Tests Declared Unsafe, Mass Recall Issued appeared initially on The Gateway Pundit
This article may have been paraphrased or summarized for brevity. The original article may be accessed here: Read Source Article.




![President Trump Gives Barron A Shout Out At Inaugural Parade: His Unexpected Response is Pure Gold! [VIDEO] president-trump-gives-barron-a-shout-out-at-inaugural-parade:-his-unexpected-response-is-pure-gold!-[video]](https://news.lateawakening.com/wp-content/uploads/2025/01/35545-president-trump-gives-barron-a-shout-out-at-inaugural-parade-his-unexpected-response-is-pure-gold-video-100x70.jpg)



