
Last month, the booster shots versus Omicron got emergency situation permission today, and a CDC panel voted to suggest the shots for individuals over the age of 12.
The most current Covid booster was allegedly reformulated to ‘safeguard’ versus Omicron subvariants.
The brand-new COVID boosters were not checked on human beings– just mice. The Food and Drug Administration is just depending on the mice trial information.
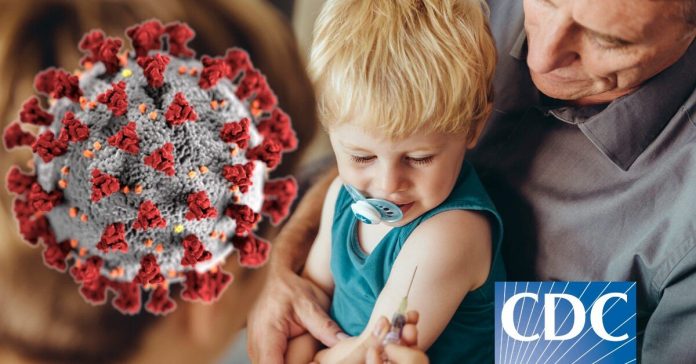
On Wednesday, the FDA licensed Moderna and Pfizer the upgraded booster shots for kids as young as 5 to 11.
From the press release:
Today, the U.S. Food and Drug Administration modified the emergency situation usage permissions (EUAs) of the Moderna COVID-19 Vaccine, Bivalent and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent to license their usage as a single booster dosage in more youthful age. The Moderna COVID-19 Vaccine, Bivalent is licensed for administration a minimum of 2 months following conclusion of main or booster vaccination in kids to 6 years of age. The Pfizer-BioNTech COVID-19 Vaccine, Bivalent is licensed for administration a minimum of 2 months following conclusion of main or booster vaccination in kids to 5 years of age.
These bivalent COVID-19 vaccines consist of an mRNA element of the initial stress to supply an immune reaction that is broadly protective versus COVID-19 and an mRNA element in typical in between the omicron alternative bachelor’s degree.4 and bachelor’s degree.5 family trees to offer much better defense versus COVID-19 brought on by the omicron version. The mRNA in these vaccines is a particular piece of hereditary product that advises cells in the body to make the unique “spike” protein of the initial infection pressure and the omicron alternative family trees bachelor’s degree.4 and bachelor’s degree.5. The spike proteins of bachelor’s degree.4 and bachelor’s degree.5 equal.
” Since kids have actually returned to school face to face and individuals are resuming pre-pandemic habits and activities, there is the capacity for increased danger of direct exposure to the infection that triggers COVID-19 Vaccination stays the most reliable step to avoid the serious repercussions of COVID-19, consisting of hospitalization and death,” stated Peter Marks, M.D., Ph.D. “While it has actually mainly held true that COVID-19 tends to be less extreme in kids than grownups, as the numerous waves of COVID-19 have actually happened, more kids have actually gotten ill with the illness and have actually been hospitalized. Kids might likewise experience long-lasting impacts, even following at first moderate illness. We motivate moms and dads to think about main vaccination for kids and follow-up with an upgraded booster dosage when eligible.”
With today’s permission, the monovalent Pfizer-BioNTech COVID-19 Vaccine is no longer licensed as a booster dosage for people 5 through 11 years of age. Both the Moderna COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine continue to be licensed for main series administration in people 6 months of age and older.
Dr. Paul Offit, MD, a leading vaccine professional and FDA consultant has alerted last month that there is inadequate proof to advise the brand-new booster shot for healthy young people and stated it might bring dangers.
During an interview with CNN, Dr. Offit stated this brand-new Covid booster shot is not likely to benefit healthy youths and stated it’s ‘unjust to make them take a danger.’
Dr. Offit: “When you’re asking individuals to get a vaccine, I believe there needs to be clear proof of advantage. And we’re not going to have medical research studies, clearly, prior to this launches, however you ‘d like to have at least human information on individuals getting this vaccine. You see a clear and significant boost in reducing the effects of prescription antibiotics, and after that a minimum of you have a correlate of defense versus BA4, BA5. Since if you do not have that, if there’s unclear proof of advantage, then it’s unfair [to ask individuals to take a danger] The advantages must be clear.”
Watch the video listed below:
FDA consultant and vaccine maker Paul Offit: “A healthy young adult is not likely to gain from a booster dosage … If there’s unclear proof of advantage, then it’s unfair to ask individuals to take a threat.” pic.twitter.com/SgBp5WZbMS
— Max Blumenthal (@MaxBlumenthal) September 24, 2022
The post FDA Authorizes Moderna and Pfizer Updated Booster Shots Against Omicron for Kids as Young as 5 to 11 appeared initially on The Gateway Pundit
This article may have been paraphrased or summarized for brevity. The original article may be accessed here: Read Source Article.




![President Trump Gives Barron A Shout Out At Inaugural Parade: His Unexpected Response is Pure Gold! [VIDEO] president-trump-gives-barron-a-shout-out-at-inaugural-parade:-his-unexpected-response-is-pure-gold!-[video]](https://news.lateawakening.com/wp-content/uploads/2025/01/35545-president-trump-gives-barron-a-shout-out-at-inaugural-parade-his-unexpected-response-is-pure-gold-video-100x70.jpg)



