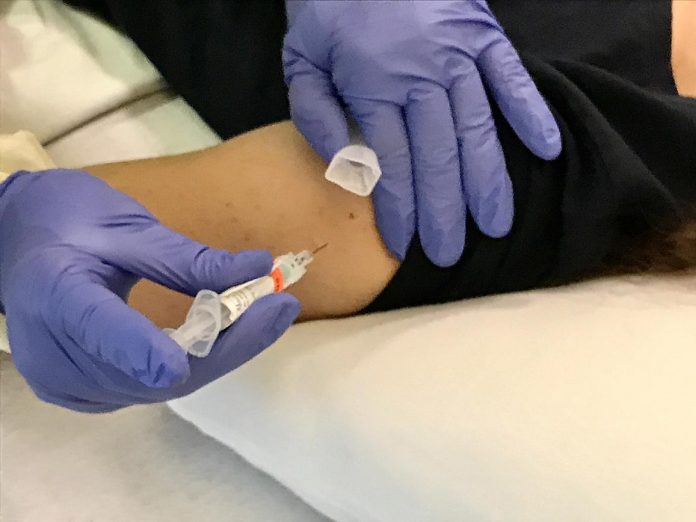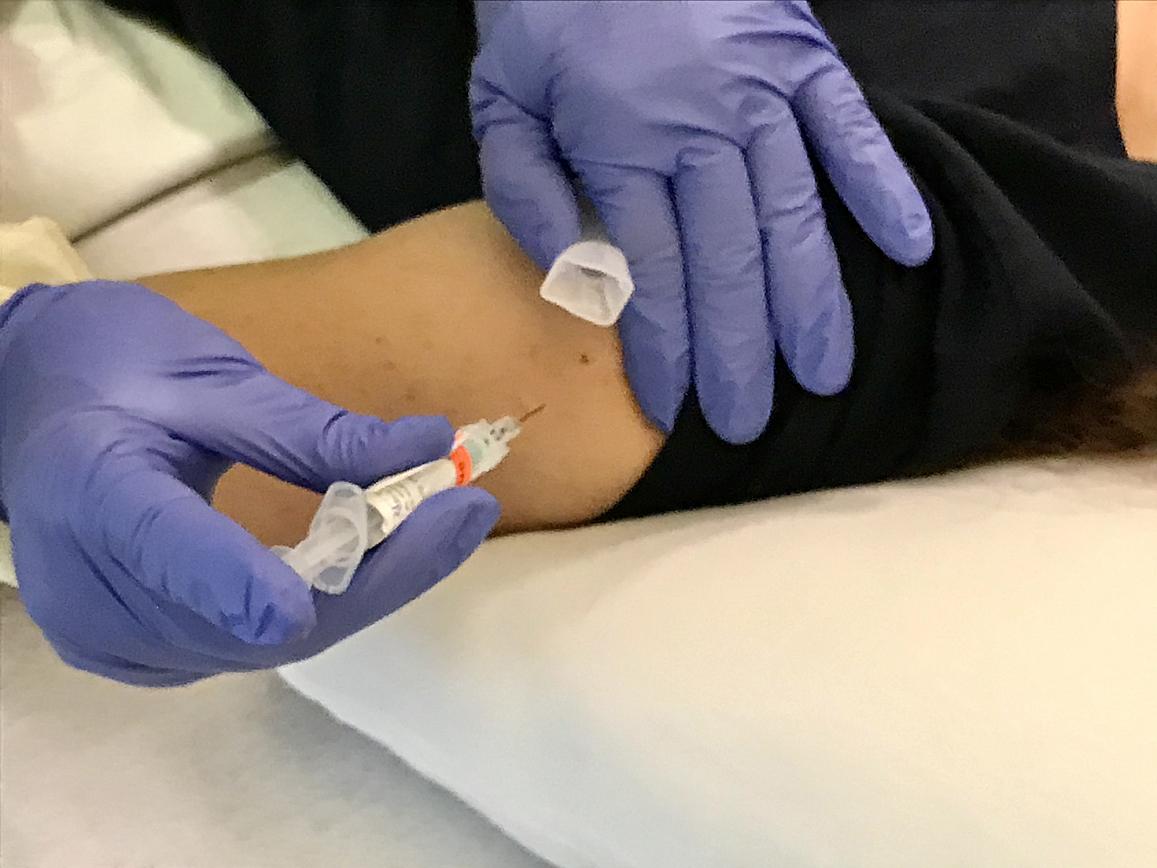
Volunteer in trial of prospect universal influenza vaccine BPL-1357 gets an intramuscular injection. NIAID
The very same innovation that was utilized to deal with COVID-19, which has actually seriously damaged countless individuals and, in the worst cases, has actually triggered death, will now be utilized to treat influenza.
The Phase 3 trial of an mRNA influenza vaccine has actually started at 2 significant pharmaceutical business, Moderna and Pfizer.
A significant Phase 3 medical trial examining the effectiveness, security, tolerability, and immunogenicity of Pfizer’s quadrivalent customized RNA (modRNA) influenza vaccine prospect in about 25,000 healthy U.S. grownups has actually started dosing individuals, the pharmaceutical giant reported last month.
” For years, there has actually been a requirement to much better address the concern of influenza, in spite of making use of existing seasonal influenza vaccines. Our experience with RNA infections and mRNA innovation has actually offered us an even much deeper understanding of the chance to possibly supply more effective vaccines that might even more lower the annual rates of the extreme results of viral illness like influenza, consisting of hospitalization and death,” declared Annaliesa Anderson, Ph.D., Senior Vice President and Chief Scientific Officer, Vaccine Research and Development, Pfizer.
” We are delighted to begin the very first Phase 3 effectiveness research study of an mRNA-based influenza vaccine that might possibly provide an enhanced influenza vaccine to assist deal with the substantial concern of this illness,” she included.
Pfizer guarantees that its medical trial individuals correctly represent the racial and ethnic variety of the nations in which they are carried out.
” The effect of influenza on racial and ethnic minority groups in the U.S. is even bigger. Black Americans are 1.8 times most likely than their white equivalents to be hospitalized for influenza while Latino and Indigenous Americans are 1.2 and 1.3 times most likely, respectively,” according to the press release
This research study is registering individuals who:
- Are at least 65 years of ages
- Are normally healthy or have steady persistent conditions
- Have not gotten an investigational or certified influenza vaccine in the last 6 months
This research study will last about 6 months. Individuals will go to the research study website a minimum of 3 times, consisting of a vaccination check out and 2 follow-up check outs to monitor their health.
Last June, Moderna reported that the very first trial individuals had actually been dosed with the business’s seasonal influenza vaccine prospect in a Phase 3 trial (mRNA-1010). Approximately 6,000 grownups from countries in the Southern Hemisphere took part in the trial.
” We are delighted to start this Phase 3 research study of our seasonal influenza vaccine prospect, mRNA-1010, our 4th mRNA vaccine prospect to start a critical Phase 3 research study. mRNA-1010 is the very first of a number of influenza vaccine prospects we are establishing with the objective of iteratively enhancing conventional vaccines by causing broad and robust immune actions. Our company believe our mRNA platform, with the versatility and speed of our production procedure, is well-positioned to resolve the considerable unmet requirement in seasonal influenza,” stated Stéphane Bancel, Chief Executive Officer of Moderna.
” Influenza vaccines are a crucial pillar in our breathing vaccine technique that consists of the advancement of mix prospects targeting several infections in a single vaccine, consisting of influenza with SARS-CoV-2 and breathing syncytial infection. With the start of dosing for its mRNA-1010 program, Moderna now has 4 programs in late phase Phase 3 research studies, including its SARS-CoV-2 booster, RSV, seasonal influenza and CMV vaccine prospects. Starting in the fall of 2022, the Company’s Phase 3 pipeline might cause 3 breathing industrial launches over the next 2 to 3 years.”
The doctor who developed the mRNA innovation consisted of in COVID vaccines is prompting the general public not to get COVID vaccination that might completely harm the crucial organs, reproductive systems, and body immune systems, or might trigger death.
The public is being deceived about the vaccine’s effectiveness and impacts, cautions Dr. Robert Malone, the Chief Medical and Regulatory Officer for The Unity Project who developed messenger mRNA rehabs in 1988.
The post Here We Go: Moderna and Pfizer Start Phase 3 Trial of mRNA Flu Vaccine appeared initially on The Gateway Pundit
This article may have been paraphrased or summarized for brevity. The original article may be accessed here: Read Source Article.