The Food and Drug Administration (FDA) has actually released an alarming caution to clients NOT to acquire more than 2 lots eye drop items being cost a few of the country’s most popular sellers. While this is certainly vital public details, this brand-new discovery must likewise raise some severe concerns about the FDA itself.
As ABC News reported Monday, the FDA in a news release Friday exposed that the eye drop items were made in a center with “insanitary conditions” and bring a “prospective danger of eye infections that might lead to partial vision loss or loss of sight.”
These items are meant to be sterilized. Ophthalmic drug items posture a possible increased threat of damage to users due to the fact that drugs used to the eyes bypass a few of the body’s natural defenses. FDA advised the maker of these items remember all lots on October 25, 2023, after firm detectives discovered insanitary conditions in the production center and favorable bacterial test arises from ecological tasting of vital drug production locations in the center.
The place of this center is unidentified at today time. The FDA did not even define if the plant’s place remained in America
According to the FDA, the affected items are marketed under the brand names CVS Health, Leader (Cardinal Health), Rugby (Cardinal Health), Rite Aid, Target Up&& Up, and Velocity Pharma. The FDA shared a complete list of the 26 items on its site

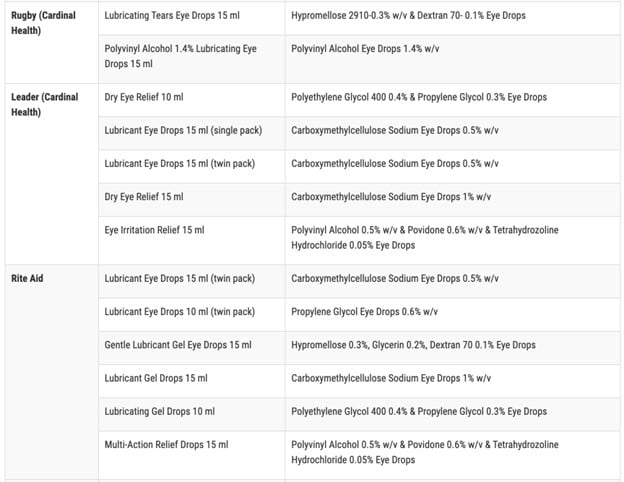
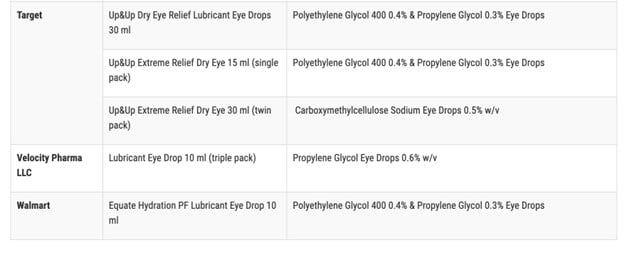
Anyone who acquired these items from these areas are advised to “right away stop utilizing” them and deal with them correctly. This consists of either dropping off the item at a drug take-back website or following the FDA’s actions to get rid of the item in the garbage according to ABC News.
The FDA states they have yet to get reports of eye infections brought on by the items, however is prompting healthcare specialists and clients to report any “negative occasions or quality issues.”
Symptoms of an eye infection consist of, “yellow, green, or clear discharge from the eye, eye discomfort or pain, soreness of the eye or eyelid, sensation of something in your eye (foreign body feeling), increased level of sensitivity to light, and fuzzy vision according to the FDA.
Target, Rite Aid, and CVS have now apparently started the procedure of getting rid of the eye drops from their shops.
Here is a video report on the story from ABC:
This is not the very first time a maker has actually offered malfunctioning and unsafe eye items to shops. The Gateway Pundit’s Alicia Powe reported in March that a minimum of 68 clients in 16 states were contaminated with Pseudomonas aeruginosa, a fatal bacterial “superbug,” thanks to neglectfulness by an India-based business called Global Pharma Healthcare.
These clients utilized Artificial Tears Lubricant Eye Drops, an item utilized to lube dry eyes. 3 individuals passed away after utilizing synthetic tears items, 8 went totally blind, and 4 needed to have their eyeballs surgically got rid of.
The CDC teamed up with the Food and Drug Administration (FDA) and state and regional health departments to examine the multistate break out. They informed clinicians and clients stop utilizing and dispose of EzriCare Artificial Tears and 2 extra items made by the very same producer, Delsam Pharma’s Artificial Tears, and Delsam Pharma’s Artificial Ointment.
Global Pharma Healthcare likewise apparently willingly remembered these very same items according to the FDA.
In August, however, the FDA released another cautioning to the American public that Artificial Tears items need to not be utilized, consisting of off-label usage in animals. This was triggered by the New Jersey Department of Health releasing a Health Alert Network message External Link Disclaimer requiring animal caretakers to right away stop utilizing EzriCare Artificial Tears, Delsam Pharma Artificial Tears, and Delsam Pharma Artificial Ointment on animal clients.
Why was this caution required if these 3 items had been remembered months earlier? Now we have lube eye drops AGAIN positioning a prospective tomb risk to the general public.
Worse, the FDA now REFUSES to call the maker accountable for these 26 items they are alerting the general public about. Is this since Global Pharma Healthcare depends on their old techniques once again and the FDA is stressed over a prospective scandal breaking out?
What is the firm concealing from the general public?
The post WHAT ARE THEY HIDING? FDA WARNING: 26 Eye Drop Products at Multiple Major Retailers Including Target Could Cause BLINDNESS– Were Manufactured in Unknown “Insanitary” Facility appeared initially on The Gateway Pundit



This article may have been paraphrased or summarized for brevity. The original article may be accessed here: Read Source Article.





![President Trump Gives Barron A Shout Out At Inaugural Parade: His Unexpected Response is Pure Gold! [VIDEO] president-trump-gives-barron-a-shout-out-at-inaugural-parade:-his-unexpected-response-is-pure-gold!-[video]](https://news.lateawakening.com/wp-content/uploads/2025/01/35545-president-trump-gives-barron-a-shout-out-at-inaugural-parade-his-unexpected-response-is-pure-gold-video-100x70.jpg)



